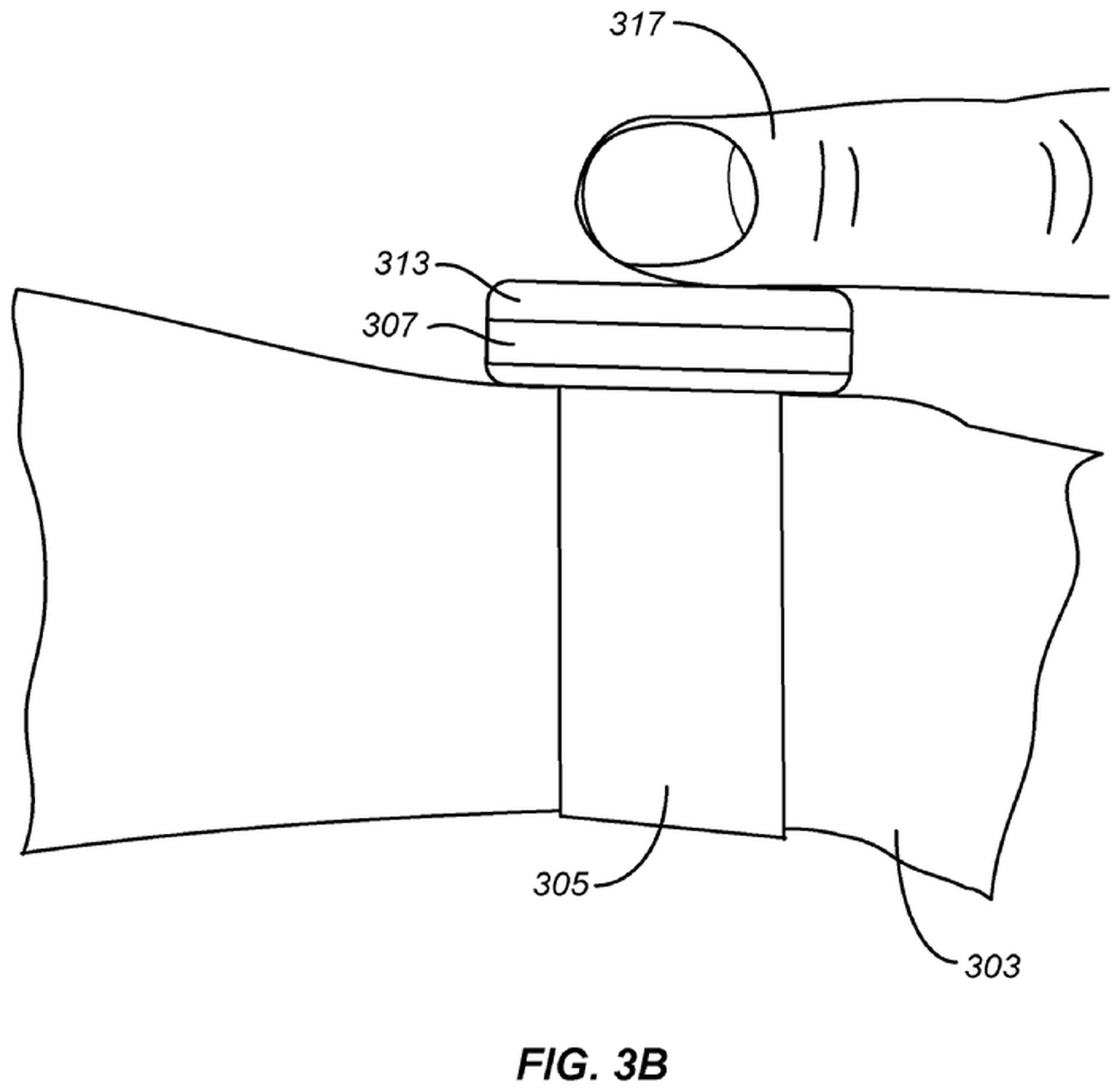
fitbit recently filed a patent application (pdf) for a force-sensitive display that would allow blood pressure readings on wearable devices. But even if the patents did guarantee success, which it doesn’t, the past few months make it hard to be confident in the future of Fitbit smartwatches.
First things first, you shouldn’t read too much into any patent application. While it can give you an idea of what a company is working on, it is a legal tool for companies to effectively call dibs on a particular innovation. In it claims section of this presentation (through storable), Fitbit describes a force-sensitive screen combined with a photoplethysmography (PPG) sensor that, when pressed, can estimate your blood pressure.
The nice thing about this concept is that it is essentially based on the traditional blood pressure cuff. Those work by cutting off the flow of blood in an artery. That pressure is then slowly released, helping doctors determine when blood flow starts again (systolic reading) and when your heart relaxes again (diastolic reading). High numbers may be a sign that your heart is working too hard to pump blood through your body.
It’s not particularly surprising that blood pressure is on Fitbit’s radar. It’s far from the first wearable to include this feature. samsung had it on your Galaxy watches for quite some time, although it uses a different mechanism that requires periodic calibration than a traditional cuff and is not available in the US for regulatory reasons. there is also the Omron Heart Guide – an FDA-cleared smartwatch where the strap doubles as an inflatable bracelet.
However, there is new momentum for cuffless, non-invasive blood pressure devices using PPG sensors. Valencell, which develops biometric sensor technology, showed up at CES 2023 with a cuffless blood pressure monitor without fingertip calibration. Last year, Movano Health, which also showed up at CES with a smart ring, announced it. functional tests completed for a radio frequency-enabled chip that could measure both blood pressure and blood glucose in wearable devices.
Fitbit’s patent is great, but its last few months have been mediocre. Its latest smartwatches, the Sense 2 and Versa 4, took a backseat to Google’s Pixel Watch. Also, features available on previous iterations of the watches, like third-party apps and the Google Assistant, are gone. Google also changed the company name to “Fitbit by Google” and recently announced that in a few years, Fitbit users will need to sign in with their Google accounts. Just this week, Fitbit experienced several server outages that left users frustrated and angry. Altogether, it doesn’t paint a pretty picture.
So it’s hard to see this type of patent filing as an exciting development. If granted, it’s more likely to appear on a Pixel Watch than on any Google Fitbit product. And that’s if this feature even sees the light of day anytime soon. While technology moves fast, healthcare technology sure doesn’t. Wearable technology companies generally veer toward “wellness” functions because they don’t require FDA regulatory oversight. However, blood pressure would probably need FDA involvement.
At best, this presentation is just more proof that non-invasive blood pressure technology is something that deeply concerns the companies of portable devices. But when and in what form it is impossible to predict. It’s also a reminder that while dreaming of life-changing healthcare technology is easy, it’s a lot harder to make it happen. By the time we see widespread wearable blood pressure technology, the Fitbit might already be a distant memory.